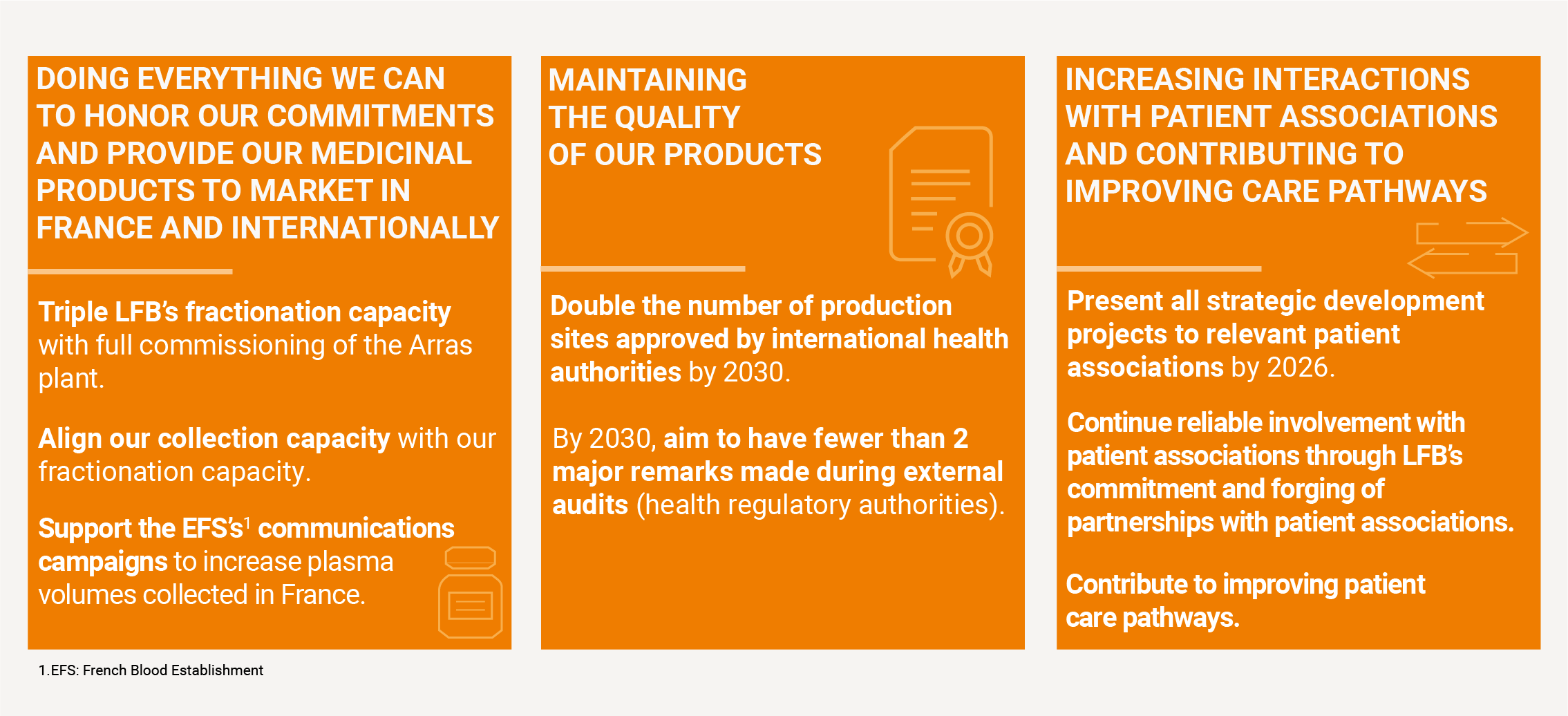
Ensuring access to treatment for patients

Serving patients: at the heart of LFB’s mission
In the face of rapidly increasing patient needs and strained production capacities, LFB has committed to ensuring equitable and safe access to treatments, both today and for future generations:
- By tripling its production capacity for plasma-derived medicinal products,
- By expanding its offerings with its own plasma collection centres,
- By helping improve patient care pathways, facilitating access to information, and ensuring better treatment monitoring, thanks to its commitment to caregivers and patient associations.
Strategic pillar of our plasma collection network
Europlasma operates 26 centres in Austria, Germany, and the Czech Republic.
With LFB American Plasma in the United States, we also have plasma and blood collection centres to meet regulatory requirements. In accordance with US FDA requirements, only medicinal products derived from plasma donated in the USA can be sold in the USA.
Our evidence:
CONTEXT FOR UNDERSTANDING THE CHALLENGES
Ensuring access to treatment in the face of rapidly growing demand
Each year, the number of patients who need treatment with PDMPs increases and therefore demand for plasma-derived medicinal products continues to rise, which puts increased pressure on available volumes.
In this context, the French government has entrusted LFB with a public health mission: to provide medicinal products derived from plasma collected by the French Blood Establishment (EFS)* to market and give priority to patients in France.
To achieve its mission, the LFB relies on an exclusive partnership with the EFS that is in charge of collection in France, with the aim of ensuring France’s health sovereignty.
In 2024, public or private plasma collection structures will only be able to cover 74% of immunoglobulins needs worldwide*. This situation requires a collective strategy to secure access to treatment for patients. Patient associations and health systems alike contribute to improving access and continuity of treatment for patients.
*Article L5124-14 of the French Public Health Code modified in 2015 by Law No. 2015-990, article 190.
Find out more about the other pillars of our CSR Strategy:
Sources:
- PPTA, 2021. Source : https://cdn.prod.website-files.com/
- Interview Jacques Brom – LFB on PharmaBoardroom.com and in Healthcare & Life Sciences Review France 2025
- https://www.ncbi.nlm.nih.gov/books/NBK591049/