LFB, expertise committed to life
News & Features
In brief

LFB, expertise committed to life
We are experts in human plasma derived therapies and an essential link between plasma donors and patients due to our expertise and commitment to patients.
Driven by a public health mission, we are a pillar of French healthcare sovereignty and an internationally recognized force in the plasma industry. We develop, manufacture and distribute biopharmaceuticals to improve patients’ lives in responsible and sustainable ways.
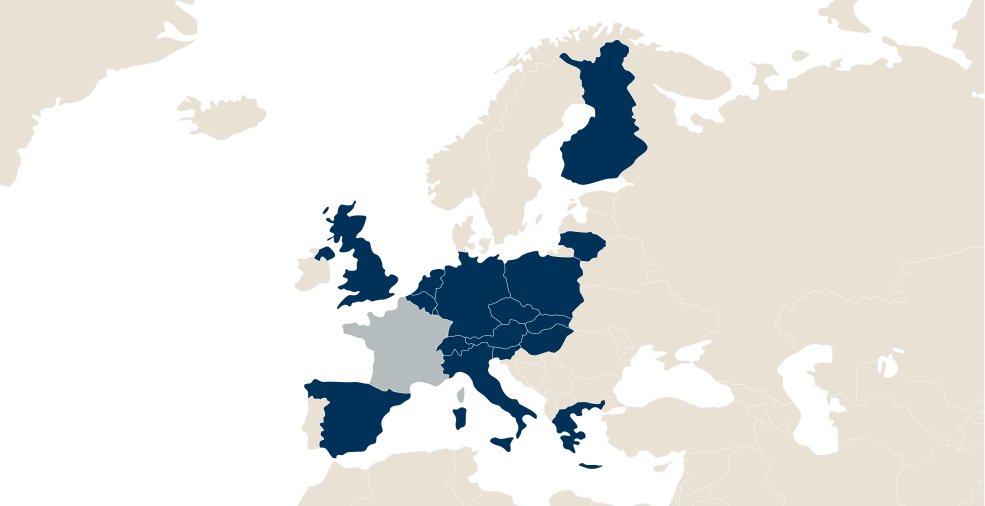
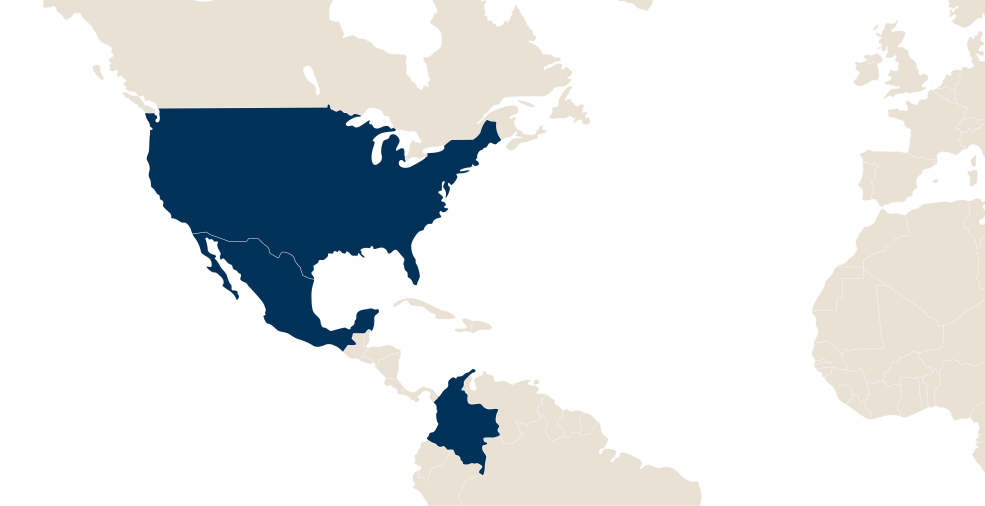
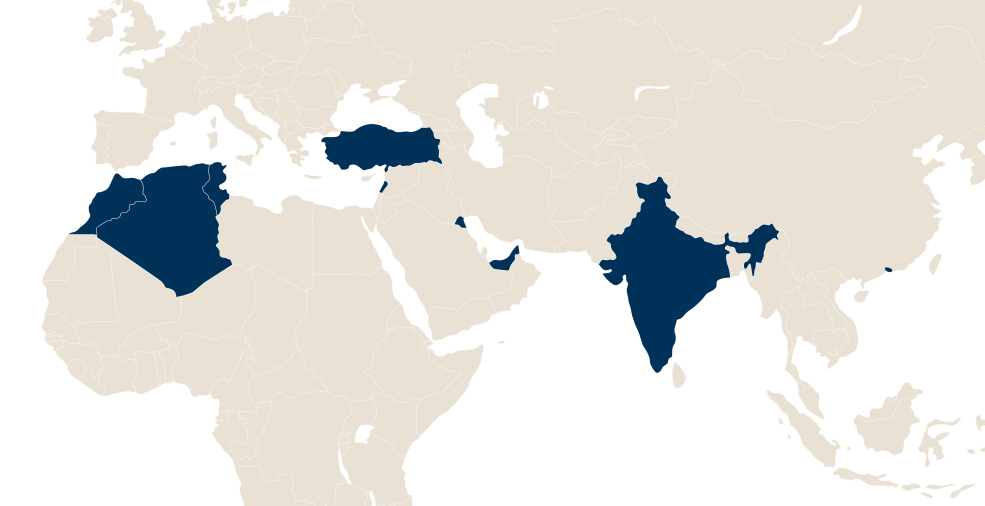
LFB in the world
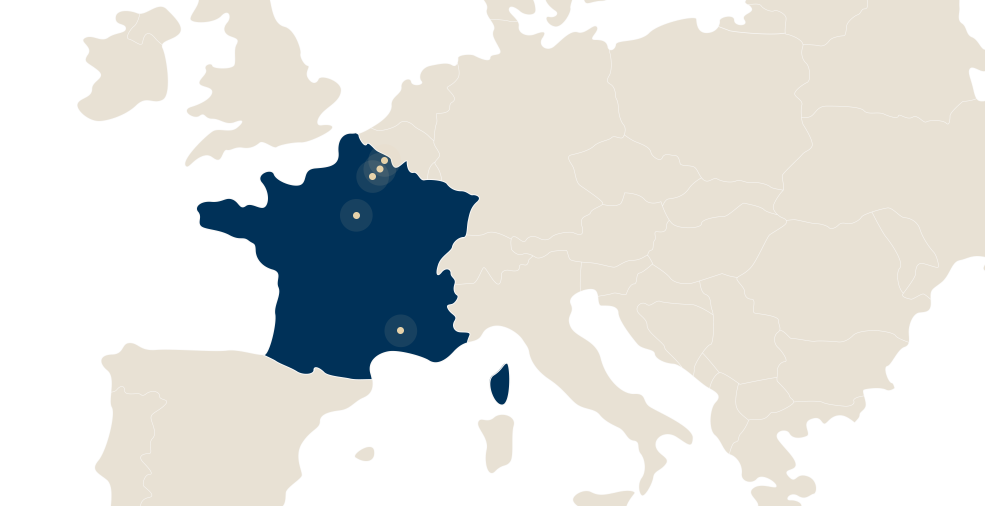
LFB has 4 sites in France:
- Alès in the Gard department,
- Arras in the Pas de Calais department,
- Les Ulis, in the Essonne department,
- Lille in the Nord department and Carvin in the Pas de Calais department