Biological safety at LFB
A pioneer in the use of nanofiltration
Since its creation in 1994, LFB has been an innovative player in the field of biological safety.
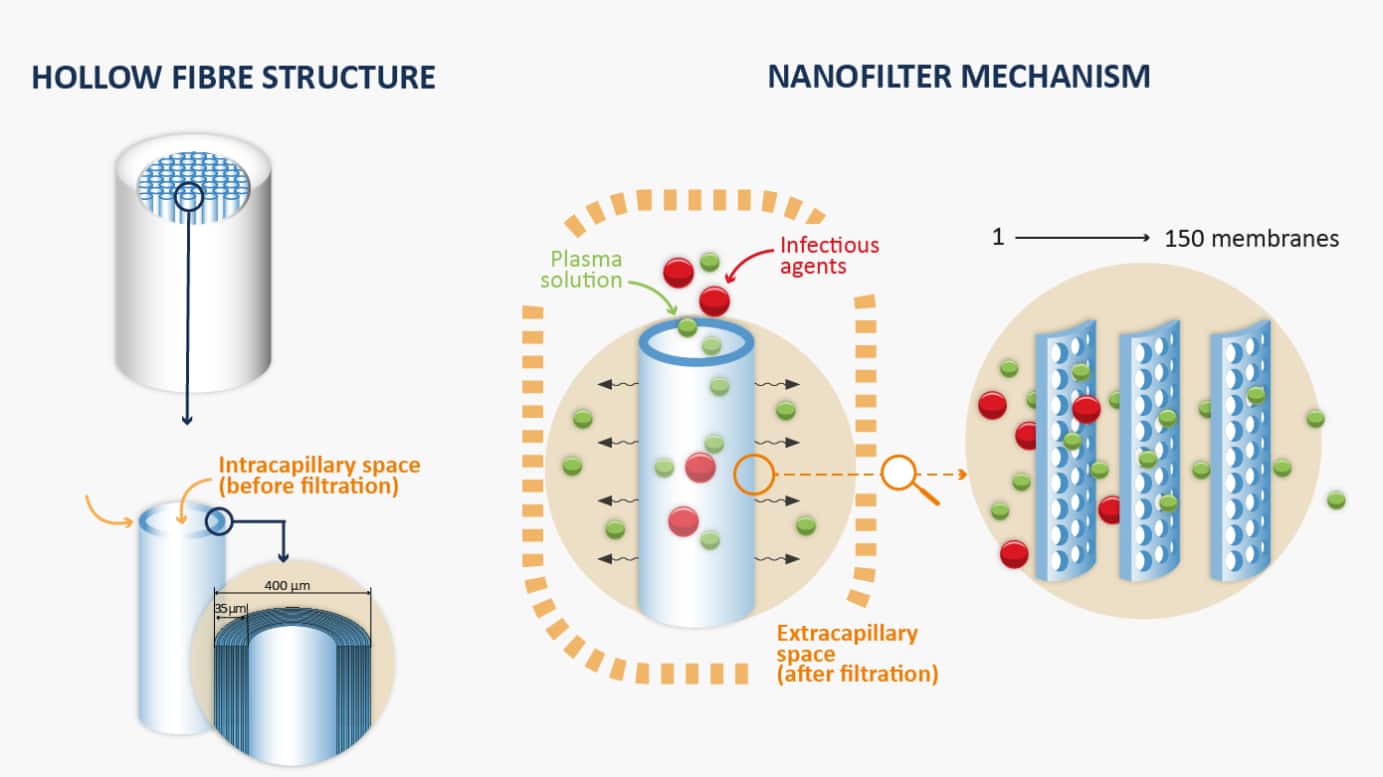
In 1995, LFB was the first pharmaceutical company to implement nanofiltration in its manufacturing processes for plasma-derived medicinal products. Nanofiltration is a technique that filters particles based on their size and has proven effective in the elimination viruses and reduction of the risk of prions.
Discover how a 35-nanometer nanofilter works:
A pioneer in ensuring the safety of the plasma before fractionation
In 1996, LFB was also the first manufacturer to implement a PCR (Polymerase Chain Reaction) technique to detect parvovirus B19 in plasma for fractionation. PCR is a nucleic acid amplification technique that is used to detect viruses before the antibodies directed against them show up in the blood. PCR is now used all plasma fractionated by LFB, to the HCV, HIV, HBV and HAV viruses, in addition to parvovirus B19.
Production steps that contribute to the safety of the medicinal product
The processes LFB uses to manufacture its medicinal products are the second line of defence in the safety system, through:
- viral inactivation and/or elimination steps with validated efficacy,
- steps to remove any prions present, whose validation was presented to the ANSM expert group in 2004,
- quality controls throughout the manufacturing process and on the finished product and compliance with specifications in force,
- compliance with Good Manufacturing Practice.
Taken together, this system helps guarantee the efficacy, safety and tolerability of the medicinal products.
Specific viral inactivation steps by physical or chemical treatment:
- Dry heating
- Pasteurisation
- pH 4 / Pepsin
- Solvent / detergent treatment.
Specific step to remove infectious agents: nanofiltration.
Manufacturing steps that contribute to the elimination and/or inactivation of infectious agents:
- Ethanol or caprylic acid precipitation,
- Chromatographies,
- Absorption on an alumina gel, precipitation, filtration,
- Depth filtration.